News
“My group uses click chemistry to study biological systems at the molecular level. We develop and exploit powerful bond-forming click reactions that enable the rapid synthesis of small functional molecules, including cancer drugs and chemical probes. We apply these novel molecular tools in multidisciplinary discovery projects spanning the fields of biology and chemistry.” – John Moses
 Cold Spring Harbor Laboratory Professor and Cancer Center member John E. Moses with Research Investigator Joshua Homer in their chemistry laboratory.
Cold Spring Harbor Laboratory Professor and Cancer Center member John E. Moses with Research Investigator Joshua Homer in their chemistry laboratory.Cold Spring Harbor Laboratory (CSHL) Professor John E. Moses and Research Investigator Joshua Homer were awarded more than $325,000 by New York State to develop a new type of antibiotic that can treat bacteria that have become drug-resistant.
New York Governor Kathy Hochul announced the award and over $15 million worth of research awards as part of the New York State Biodefense Commercialization Fund on April 11, 2022. According to the World Health Organization, resistant bacteria and other microbes are one of the greatest threats to public health.
Antibiotic resistance occurs when bacteria or other germs develop the ability to resist the drugs designed to kill them, usually through mutations. “Drugs are typically rigid structures that bind to targets within the bacteria in a precise way,” says Homer. “Small changes in a bacterial drug target can render the drug useless.”
Moses and his team are reworking antibiotic drugs to have a “shape-shifting” component that can adopt different configurations similar to a Rubik’s Cube. Moses says, “The drug molecule, although still acting like the original antibiotic, now has enough flexibility to overcome the small structural changes employed by the bacteria to achieve resistance.”
Charles Prizzi, CSHL Senior Vice President, Advancement, says, “The integration of chemistry into our life science research is an exciting area of investigation which is now being applied to public health threats like antibiotic resistance.”
Written by: Eliene Augenbraun, Creative Director | publicaffairs@cshl.edu | 516-367-8455
 Joshua Homer, a postdoctoral researcher in Professor John E. Moses’ group at Cold Spring Harbor Laboratory, seen drawing out a “click” chemistry reaction. Click reactions allow chemists to assemble molecules quickly, reliably, and cleanly.
Joshua Homer, a postdoctoral researcher in Professor John E. Moses’ group at Cold Spring Harbor Laboratory, seen drawing out a “click” chemistry reaction. Click reactions allow chemists to assemble molecules quickly, reliably, and cleanly.They say you can’t teach an old dog new tricks, but can you do new tricks with old chemical catalysts? Cold Spring Harbor Laboratory Professor John E. Moses and his team have paired a catalyst called “Barton’s base” developed in the 1980s with a new family of chemical reactions. This powerful combination results in a chemical transformation that happens within minutes or even seconds. Their new chemistry can accelerate the generation of complex molecules needed for biomedical research, drug development, and materials science.
Click chemistry enables chemical building blocks to be joined together quickly, reliably, and cleanly. A click reaction often requires a catalyst to jumpstart it. Barton’s base is so powerful that Moses only needs a small trace of it to spark a reaction. Moses says, “Reducing the amount of catalyst needed for a reaction is cost-effective and beneficial to the environment.”
The new reaction, called accelerated SuFEx click chemistry (ASCC), also allows multiple steps to be skipped in some synthetic pathways, including the synthesis and purification of intermediate molecules. Moses says, “Removing steps has a huge influence on the amount of waste generated from a reaction. The ASCC method will greatly enhance the ‘green credentials’ of SuFEx click chemistry, especially when applied on an industrial scale.”
Moses feels a personal connection to Barton’s base. He says, “Sir Derek Barton, who first prepared Barton’s base in the 1980s, happens to be my academic grandfather.” Barton trained Moses’ Ph.D. advisor Sir Jack E. Baldwin at the University of Oxford. Following in the footsteps of his academic ancestors, Moses and his team continue to come up with more and more new chemistry tricks.
Written by: Luis Sandoval, Communications Specialist | sandova@cshl.edu | 516-367-6826
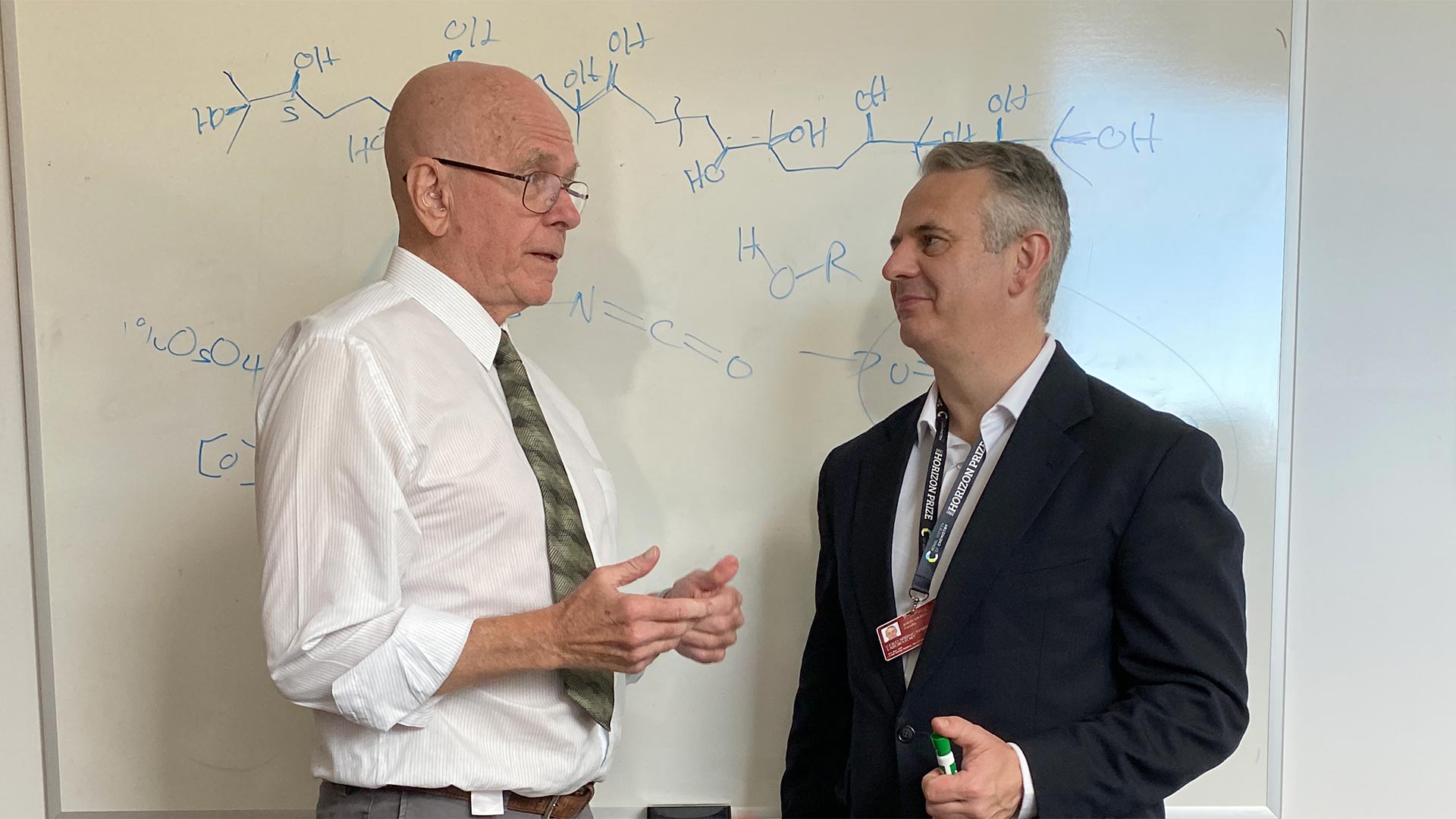 Cold Spring Harbor Laboratory Professor John E. Moses (right) first started doing click chemistry as a postdoc in K. Barry Sharpless’ (left) lab at Scripps Research. Click reactions, like the one drawn on the board behind them, happen very quickly and efficiently. Cleverly designed reaction partners rely on catalysts to help them snap together like LEGO® bricks. With families of reaction partners, the Moses lab can create hundreds of tweaks to old drugs or develop completely new materials, including polymers.
Cold Spring Harbor Laboratory Professor John E. Moses (right) first started doing click chemistry as a postdoc in K. Barry Sharpless’ (left) lab at Scripps Research. Click reactions, like the one drawn on the board behind them, happen very quickly and efficiently. Cleverly designed reaction partners rely on catalysts to help them snap together like LEGO® bricks. With families of reaction partners, the Moses lab can create hundreds of tweaks to old drugs or develop completely new materials, including polymers.Moses’ need for speed landed him at K. Barry Sharpless’ lab at Scripps Research as a postdoc. “Click chemistry” is the term Sharpless, a Nobel Prize–winning chemist, devised to describe a particular class of fast, reliable, and high-yielding reactions. Click chemists use cleverly designed catalysts to cause pairs of molecules to assemble—click together—like LEGO® bricks. The reactions are not only fast, but they are also specific. And they are changing how cleanly and efficiently drugs and other materials can be made.
CSHL Professor John E. Moses and collaborator Nobel laureate K. Barry Sharpless show click chemistry in action, synthesizing a helical polymer in about 10 minutes.
Fast chemistry
One of the biggest obstacles to making any molecule is the speed of the reaction. Catalysts, including enzymes in biological systems, make slow reactions happen faster. Moses developed click chemistry using sulfur and fluoride exchanges, or SuFEx reactions, plus a catalyst, to link small, versatile molecules to other reaction partners. These click reactions happen very quickly—in some cases, almost instantly.
To make more complex molecules, chemists string together a series of reactions. Moses and collaborators have reengineered production methods for a few important, older molecules like the antibiotic vancomycin. In other cases, they are inventing a broad spectrum of useful new molecules.
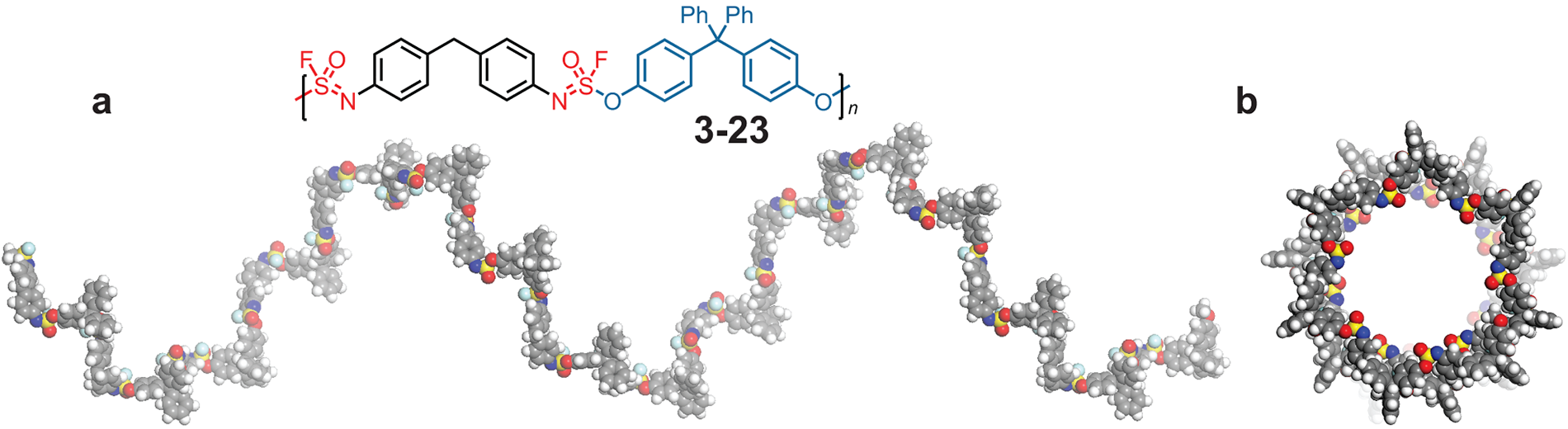
One exciting product is a polymer: long strings of identical units hooked together one after another. Natural polymers include silk, wool, and plant fibers. Plastics and synthetic rubber are synthetic polymers.
Making a non-polymer requires two molecules that fit together; when their pairing is finished, there are no more reaction sites left for that kind of reaction. But add a second reaction site to each molecule (or “monomer”), and the reaction can lead to a string of monomers linked together like a chain. Moses reported in Nature Chemistry on a versatile new polymer his lab and collaborators invented that takes less than a day to make. The team designed monomers with a third reaction site available to add side chains in later reactions, yielding a polymer decorated along its chain like a Christmas tree.
Dirty reactions add up
Click reactions may be fast, but if the reactions do not use up all the starting ingredients, they are full of unwanted side products, or yield few of the right molecules. The time saved in making the reaction will be lost in purifying the product. For simple reactions, a few percentage points of material ending up as byproducts or leftovers, may not constitute a problem. But many drugs require a series of reactions, and leftovers quickly add up. Not only that, but the solvents used to purify desired products can be bad for the environment.
Fortunately, click chemistry is so efficient that most of the ingredients end up making the product and leaving little waste. That is because click reactions are designed to involve very willing reaction partners. The force driving the reaction is so strong that the molecules will nearly always bond the same way, produce the wanted materials, and leave behind little unused or misused reactants. Purifying a product can require organic solvents, but click reactions are designed to work in water or nontoxic solvents, making these reactions less toxic to the environment.
Teaching old drugs new tricks
Moses and other click chemists are developing families of reaction partners. For example, with antimicrobial resistance becoming an increasing problem, Moses and his team are making new antibiotics out of old ones. In 2020, they reported generating nearly 300 new candidates in the journal Angewandte Chemie. They had made small changes to a library of potential drugs—some showed activity against the dangerous drug-resistant bacterial infection MRSA (Methicillin-resistant Staphylococcus aureus).
There are many diseases for which there are few or no treatments available. The Moses lab is working on developing a treatment for one such disease: leprosy. Joshua Homer, a chemist and postdoc in the Moses lab, recently received a $150,000 grant from the New York Community Trust to create new drugs using a rapid click chemistry approach. Moses notes how hard it has been to come up with a good drug “because leprosy is very slow-growing. It takes a long time to kill it because it doesn’t replicate very fast.” That means people with the disease need to take a drug for a long time. Homer says, “drug treatments that are available have some pretty severe side effects, like skin discoloration for one. Hopefully, we’ll get some derivatives that are better at treating leprosy and reducing some of those side effects.” They designed 200 new versions of three previous leprosy drugs and are currently testing them for efficacy.
Moses hopes to keep teaching old drugs new tricks. He is excited to develop new drugs with his biology colleagues at CSHL, saying:
“We bring materials that they [biologists] can’t get, they can’t access. It’s as simple as that. Things that have never been created in the universe before. They look pretty, but what do they do? How do you find out? We can give them to a biologist, who has an anti-microbial assay, or a cancer expert who has a cell-based assay.”
With back and forth between large libraries of chemical tweaks and cutting-edge biological approaches, CSHL is poised to invent and test many new molecules which may have therapeutic applications.
Editor’s note
John E. Moses joined the CSHL faculty in 2020 as the first chemist in the Laboratory’s 130-year history. He did his Ph.D. (DPhil) at the University of Oxford. Moses began working on click chemistry as a postdoc in Nobel laureate K. Barry Sharpless’ lab at Scripps Research. The chemistry between Sharpless, Moses, and the click chemistry community seems pretty unstoppable. For their innovative work, the group won the first-ever Organic Division Horizon Prize: the Robert Robinson Award in Synthetic Organic Chemistry from the Royal Society of Chemistry.
Written by: Luis Sandoval, Communications Specialist | sandova@cshl.edu | 516-367-6826
“Click chemistry” is the term Nobel Prize–winning chemist K. Barry Sharpless coined to describe a particular class of fast, reliable, and green reactions. These reactions allow molecules to click together like LEGO® bricks. Cold Spring Harbor Laboratory (CSHL) Professor John E. Moses first studied click chemistry in Sharpless’ lab at Scripps Research. He is the Laboratory’s first professor of chemistry. Moses is using click chemistry to create new molecules, including improved drugs and materials.
Watch Moses, Sharpless, and Joshua Homer, a postdoc in the Moses lab, demonstrate a click reaction, synthesizing a polymer in minutes.
Read the related story: Unstoppable chemistry
F.M. Kirby Foundation donates $115K for chemistry research.

From left to right: Charlie Prizzi (CSHL Advancement senior vice president & special advisor to the President), Diana Kostas (secretary and treasurer, F.M. Kirby Foundation), Justin Kiczek (executive director, F.M. Kirby Foundation), and CSHL Professor John E. Moses
On September 16th, 2021 the F.M. Kirby Foundation donated $115,000 to support chemistry research at Cold Spring Harbor Laboratory (CSHL) Professor John E. Moses’ laboratory. Moses is a leader in click chemistry and how to synthesize new molecules useful for drugs, biological tools, and material science. Click chemistry uses a unique type of reaction that fuses or “clicks” molecules together in an efficient and environmentally safe way.
The F.M. Kirby Foundation is a non-profit that invests in the future of communities. They support a broad range of organizations in education, health, environmental conservation, arts and humanities, religion, public affairs, and human services. The Foundation has donated a total of $1.3 million to CSHL since 2006. In 2020, the Foundation donated $115,000 to the Moses laboratory to purchase and install a nuclear magnetic resonance (NMR) machine. The NMR machine helps the lab decipher the structure of the novel chemicals they synthesize.
Written by: Luis Sandoval, Content Developer/Communicator | sandova@cshl.edu | 516-367-6826
 Special Feature – June 2021
Special Feature – June 2021
This week has been a fruitful one for the newly forged chemistry department at Cold Spring Harbor Laboratory. Following the announcement of Prof. John Moses’s win of the RSC 2021 Organic Division Horizon Prize, postdoctoral fellow Dr. Joshua Homer of the Moses Laboratory has been awarded a grant by the New York Community Trust to support his endeavors in the development of new and improved leprosy treatments.
The Heiser program, established by the will of Dr. Victor Heiser, an American physician and researcher who spent his professional life studying and caring for people with leprosy, provides fellowships for early-career researchers as well as seminal research projects led by senior scientists to improve the diagnosis and treatment of leprosy. Dr. Joshua Homer will use state-of-the-art modular click-chemistry techniques to develop and test unprecedented numbers of novel drug analogues to improve on the current front-line treatments and explore unexplored therapeutic space.
This work will be conducted primarily at Cold Spring Harbor Laboratory, where the merger between chemistry and biology will allow for this ground-breaking work to flourish. With mentorship from Prof. John Moses (CSHL), a world-leader in the field of click chemistry, and collaboration with Dr. Ramanuj Lahiri at the National Hansen’s Disease Program, Louisiana, an expert in leprosy infections, the new drugs developed from this work will change the lives of those affected by this debilitating disease.
Winner: 2021 Organic Division Horizon Prize:
Robert Robinson Award in Synthetic Organic Chemistry
June 2021
For the development of multidimensional click chemistry, a next-generation click-technology that extends bond creation into the three-dimensional world, opening doors to new frontiers in biomedicine, materials science, and beyond.
 Combining Chemistry and Biology at CSHL
Combining Chemistry and Biology at CSHL
October 1, 2020
Professor John Moses joins the CSHL faculty, specializing in the field of click chemistry.
 Cold Spring Harbor Laboratory to open first chemistry lab in its 130-year history
Cold Spring Harbor Laboratory to open first chemistry lab in its 130-year history
October 2020
Renowned molecular biology institute is bringing click chemistry to drug discovery



